Targray Cathode Materials for Li-ion Battery Manufacturers
There are multiple cathode materials to choose from within the Li-ion battery space. Originally, the primary active component of the cathode was cobalt. Today, cobalt is frequently being substituted out partially with nickel (NMC, NCA). Cathode materials require extremely high purity levels and must be almost entire free of unwanted metal impurities – notably iron, vanadium and sulfur.
Targray offers a complete portfolio of high-performance, high-capacity cathode materials which have been used by Argonne National Laboratory to achieve unprecedented battery performance. These include Nickel Cobalt Aluminum (NCA), Core Shell Gradient (CSG), Spinel-based lithium-ion (LMO), Cobalt-based lithium-ion (LCO) and Nickel Cobalt Manganese (NCM or NMC).
Cathode Binders
Our portfolio of binder materials includes Styrene Butadiene Copolymer (SBR), and Polyvinylidene Fluoride (PVDF), used in the cathode and anode electrode slurry making process for Lithium-ion batteries. Binders such as SBR and PVDF hold the active material particles together and in contact with the current collectors i.e. the Aluminum Foil (Al foil) or the Copper Foil (Cu foil).
Aluminum Foils
A rolled foil (RA-type), made from wrought Cu is generally used for high-energy, high-power applications. Aluminum foils are used as the cathode current collector of secondary Li-ion batteries. Currently, the anode is comprised of a Graphite mixture, while the cathode combines Lithium and other choice metals, and all materials in a battery have a theoretical energy density. With Lithium-ion, the anode is well optimized, and design changes will yield little to no significant improvements in performance. On the other hand, the cathode material is wide open to enhancements, and explains why today’s battery research is so heavily focused on this area.
Cathode Active Materials
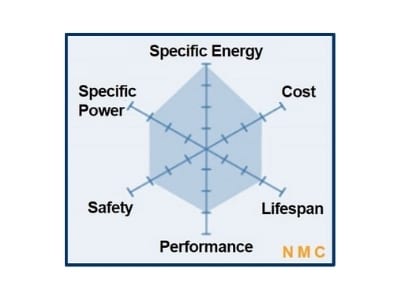
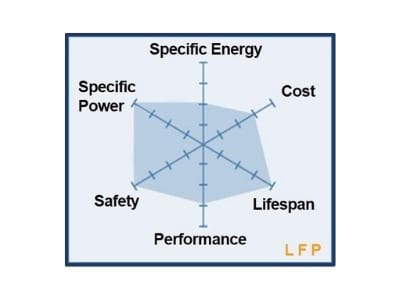
Cathode Active Materials are the main elements dictating the differences in composition while building positive electrodes for battery cells. The cathode materials are comprised of cobalt, nickel and manganese in the crystal structure forming a multi-metal oxide material to which lithium is added. This family of batteries includes a variety of products that cater to different user needs for high energy density and/or high load capacity.
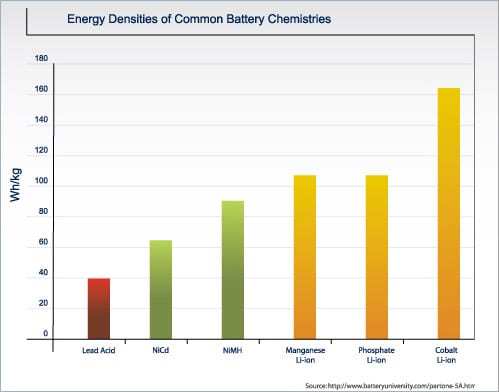
| Chemistry | Nominal V | Charge V limit | Charge & Discharge C-rates | Energy Density Wh/kg | Applications |
|---|---|---|---|---|---|
| Cobalt | 3.60V | 4.20V | 1C limit | 110-190 | Cell phone, cameras, laptops |
| Manganese (spinel) | 3.7-3.80V | 4.20V | 10C cont. 40C pulse | 110-120 | Power tools, medical equipment |
| NCM (nickel-cobalt manganese) | 3.70V | 4.10V* | ~ 5C cont. 30C pulse | 95-130 | Power tools, medical equipment |
| Phosphate | 3.2-3.30V | 3.60V* | 35C cont. | 95-140 | Power tools, medical equipment |
* Higher voltages provide more capacity but reduce cycle life
Working closely with our supply partners and organizations including the Electrochemical Society and NAATBatt International, we help battery manufacturers and researchers worldwide commercialize ambitious new technologies for the energy storage market.